Gel Electrophoresis: An easy tutorial and the simplest way to find the isoelectric pH of a protein molecule
The term electrophoresis is decomposed into two main words i.e., electro and phoresis. Electro comes from electricity while phoresis is derived from phorus which means carried by. Thus, electrophoresis is an analytical separation technique, specifically applied for the analysis of charge-bearing species. It is performed on a gel flatbed. The charged particles migrate on this gel bed under the influence of an applied electric field and are separated based on their shape, particle size, and magnitude of charge. So here is our review on “Gel Electrophoresis: An easy tutorial and the simplest way to find the isoelectric pH of a protein molecule”.
Author
Ammara Waheed

ResearchGate : Click here to see Ammara‘s profile
In this article, we have penned down the steps to perform gel electrophoresis using visual illustrations. You will also find an easy trick for calculating the isoelectric pH of a protein molecule, which means you would definitely not want to miss this article.
Therefore, you need to do a simple thing i.e., continue reading until the end.
Understanding Gel Electrophoresis
Gel electrophoresis works on an electrokinetic principle. A gel electrophoretic system is composed of a stationary phase and a wet mobile phase in an electrolytic cell. Two electrodes of opposite charge called the cathode, and the anode are present, connected by an electrolyte.
Negatively charged fragments from a complex sample mixture travel towards the positively charged anode and vice versa for positively charged fragments. Shorter molecules move faster and farther than the longer ones. In this way, analytical separation occurs.
What is the stationary phase in gel electrophoresis?
A porous gel matrix is used as a stationary phase in gel electrophoresis such as the agarose or polyacrylamide gel. The gel is a cross-linked (mesh-like) polymer whose composition and pore size are varied depending upon sample requirement. The gel molecules are held together by hydrogen bonding which results in tiny pore formation within the gel.
Agarose gel has a large pore size; it is mainly used for the separation of nucleic acids and large proteins (larger than 200 kDa). The polyacrylamide gel is a more common stationary phase for the separation of small proteins via gel electrophoresis. Partially hydrolyzed starch can also be used as a gel medium for electrophoresis. In all cases, a horizontal plate is used for holding the gel.
What is the mobile phase in gel electrophoresis?
Buffer solutions of varying pH are used as mobile phase solvents for sample preparation in gel electrophoresis. The tris-acetate-EDTA (ethylenediamine tetra-acetic acid) and the tris-borate-EDTA buffer solutions are the most popular buffer choices in gel electrophoresis.
Steps to perform gel electrophoresis for a DNA separation
Step I: Gel packing
The agarose powder is first heated by adding the respective buffer. Upon cooling, a solid, squishy gel of agarose is obtained. The electrophoretic plate is then filled with this gel. Indentations called channels, lanes, or wells are created in the gel plate with the help of a fibrous comb.

Step II: Sample loading
The DNA sample prepared in a buffer solution is then loaded into these wells.

Step III: Electric field application
An external electric field is applied to convert the electrophoretic system into an electrolytic cell. This converts the gel into a running medium.

Step IV: Analyte separation
The DNA molecules are negatively charged due to the negatively charged phosphate (PO43-) groups present in their structure. Thus, negatively charged DNA molecules migrate toward the positively charged anode.

As the sample mixture consists of differently-sized fragments of DNA so smaller molecules travel faster and farther in the gel medium. In contrast, the larger molecules stay behind. The separation of DNA fragments thus occurs in this way.

Step V: Identification
The fragmentation pattern obtained in Step IV is compared to the standard fragmentation patterns available and the unknown sample is identified. The gel plate can also be stained with certain chemical visualizing agents to make the DNA fragments visible. Ethidium bromide (C21H20BrN3) is often used as a visualizing agent. It intercalates into the DNA fragments and acts as a fluorescent tag so that the fragmentation pattern becomes visible under UV light as bands.

Steps to perform gel electrophoresis for a protein separation
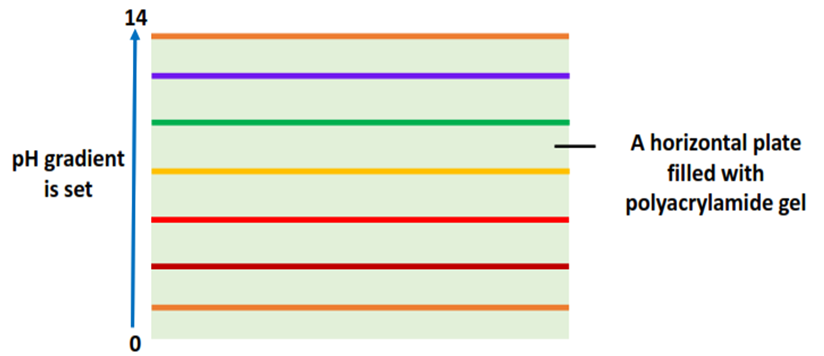
Step I: Gel Packing
The electrophoretic plate is filled with the polyacrylamide gel. A pH gradient from 0-14 is set across the plate with the help of the buffer solution poured into the gel plate.
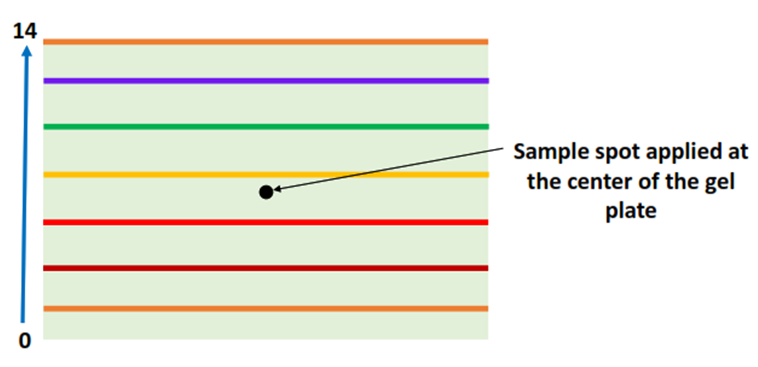
Step II: Sample preparation and loading
The protein sample is applied at the center of the gel-filled plate with the help of a micropipette.
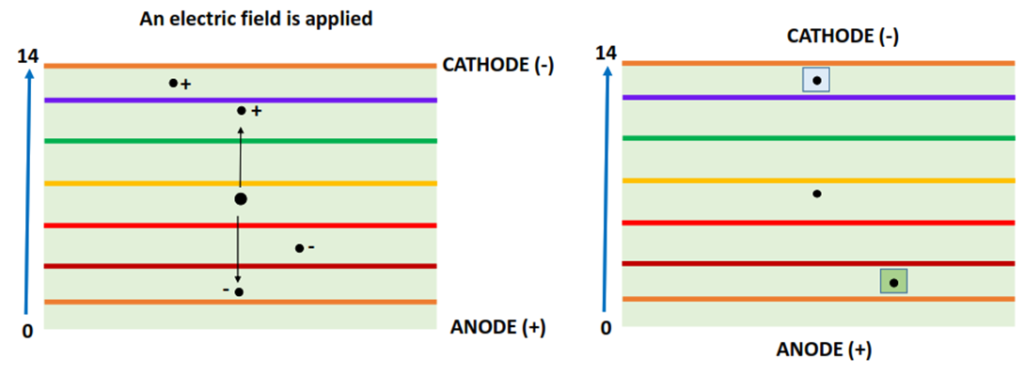
Step III: Electric field application
An external electric field (approx. 100 volts) is applied that converts the electrophoretic plate into an electrolytic cell.
Step IV: Analyte separation
Proteins are macromolecules made up of a large number of amino acids. Charged amino acids in the protein structure lead to an overall charge present on the protein molecule.
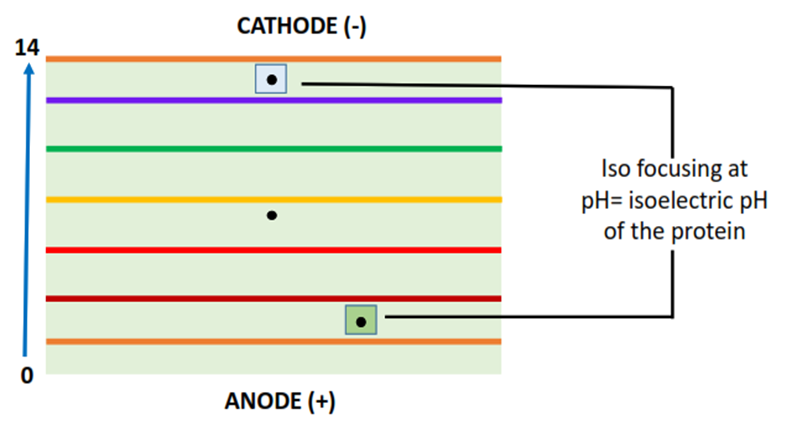
The positively charged protein molecules travel towards the negatively charged cathode while the negatively charged protein molecules migrate towards the positively charged anode (as shown above). When a protein reaches its isoelectric pH, it stops moving and is iso-focused, resulting in the desired protein separation.
The speed/ velocity (v) at which the charged protein molecules travel is the product of their mobility (m) and the applied electric field (E). v= m x E. The mobility (m) depends on particle shape, molecular size, charge, and temperature of the system.
Step V: Collection and quantification
The iso-focused protein molecules are either separately scratched out and extracted from the gel plate, collected and quantified as a final step. Contrarily, the gel as a whole can be transferred to a solid support and probed with specific antibody molecules for protein quantification. This method is known as Western blotting.
Isoelectric focusing of proteins in gel electrophoresis
The isoelectric pH of a protein is the pH at which the polarity of different functional groups present in the protein molecule is equally canceled out. This gives the protein molecule a net zero charge. Thus, the protein stops moving on the gel plate. This phenomenon is called isoelectric focusing or simply iso-focusing.
The simplest method to find the isoelectric pH of a protein molecule
A protein molecule based on three different amino acids i.e., glycine, lysine, and aspartic acid is shown below.
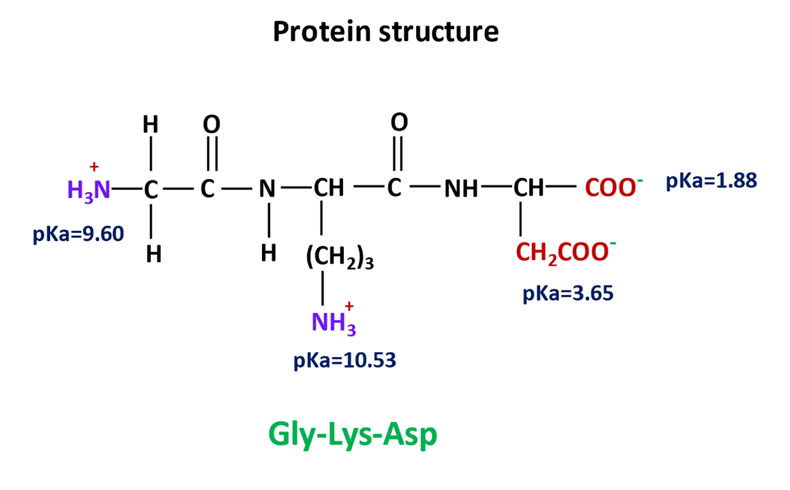
The amino acids consist of charged carboxyl and amino functional groups. All these groups have a specific pKa value.
To calculate the isoelectric pH of this protein molecule, you can follow the simple steps given below:
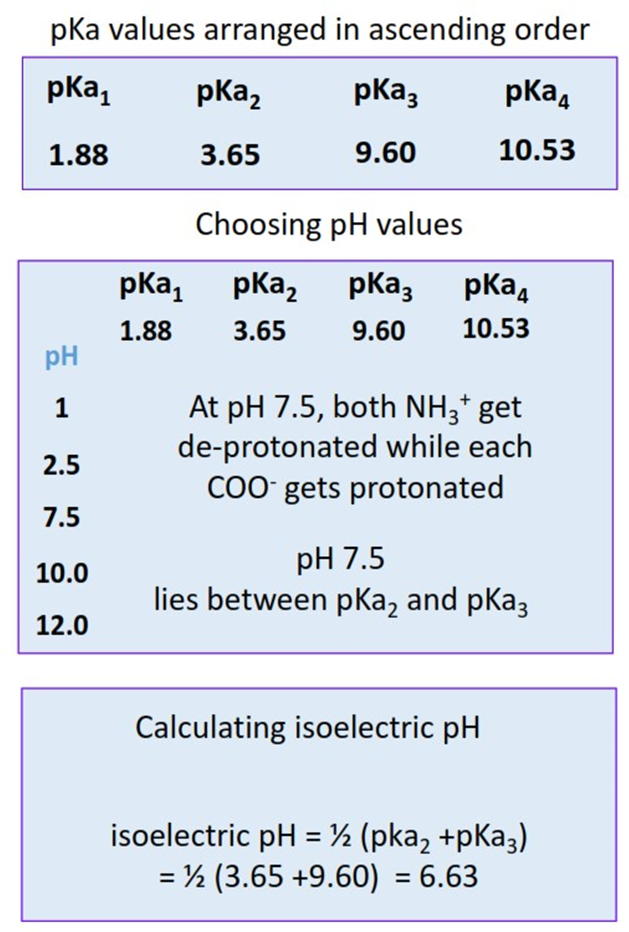
- Step I: Arrange all the pKa values in an ascending order.
- Step II: Choose five different pH values such that one pH value is lower than the lowest pKa. One pH is higher than the highest pKa, and three pH values are such that they lie in between pKa1, pKa2, pKa3,and pKa4 respectively.
- Step III: Calculate the net charge present on the protein molecule at each selected pH.
- Step IV: Note the pH at which the net charge calculated is equal to zero. Take the average of the two pKa values in between which this pH lies. This is the isoelectric pH for the protein i.e., the pH at which it will be iso-focused on the electrophoretic gel plate.
Gel electrophoresis for protein separation is usually performed in one dimension. On the other hand, DNA fragmentation through gel electrophoresis is often carried out as a two-dimensional protocol. 2-D electrophoresis allows faster separation of molecules in a large bulk.

Applications of gel electrophoresis
- Gel electrophoresis is extremely valuable in the forensic industry. Recognizing DNA fragmentation patterns help resolve crimes.
- Nucleic acid electrophoresis is widely applied for genome sequencing and DNA fingerprinting. It can also be used for parental recognition.
- Microbiologists study and invent new drugs and gene therapies using gel electrophoresis.
- Gel electrophoresis is important in medical diagnosis and for the detection of food contaminants.
- Protein-specific electrophoresis holds primary significance in immunology.
Conclusion
Considering the versatile applications of gel electrophoresis in multiple scientific domains, we must appreciate its value as a simple analytical technique that allows rapid separations with high sensitivity.
Also Read: The Fascinating World of Ionic Liquids & Their Usage in Liquid-Liquid Chromatography
Follow Us on