Comparison of Conventional Verses Green Synthesis of Nanoparticles
Author
Hajira Mahmood
Post Designer
Aqsa Iqbal

Green Synthesis of Nanoparticles. The goal of green nanoparticle synthesis is to use sustainable technologies and produce as little waste as possible. Recently, the development of nanotechnology has placed a strong emphasis on green methods that use nontoxic precursors and mild reaction conditions to promote environmental sustainability. Globally, nanotechnology is becoming a vast field of study spanning multiple disciplines. Because of their distinct physical and chemical characteristics, nanoparticle design, production, characterization, and applications have drawn more attention. It produces more stable materials and is easy, affordable, and reasonably repeatable. The green synthesis process does not require hazardous chemicals, high temperatures, high pressures, or energy.
Nanoparticles
In general, nanoparticles are incredibly small particles that range in size from 1 to 100 nm and have entirely different properties from bulk materials. The efficiency of a nanoparticle is proportional to its size; the smaller the particle, the more efficient it is. Their greater surface area to volume ratio results in variations in size, distribution, and shape, among other unique features. The enhanced catalytic and biological characteristics of Ag nanoparticles are due to their larger surface area. Because of their amazing physicochemical, optical, and biological qualities, noble metal nanoparticles such as gold, silver, palladium, and platinum are widely used in a variety of industrial and therapeutic applications. Because of their potential uses in food and health, including antibacterial and anticancer activities, silver nanoparticles are currently quite popular. Many methods have been used to create these nanoparticles, but green synthesis has gained more significance because it produces no harmful byproducts. Plant components have been used to create green synthesis.
Applications of Silver Nanoparticles
Because of their antimicrobial qualities, silver nanoparticles have found extensive use in the medical field, food storage, textile coatings, and a variety of environmental applications. Because of their special electrical, optical, and biological qualities, nanoparticles are used in drug delivery, biosensing, imaging, catalysis, the creation of nanodevices, and medicine.

Comparison of Traditional Methods to Green Synthesize Nanoparticles
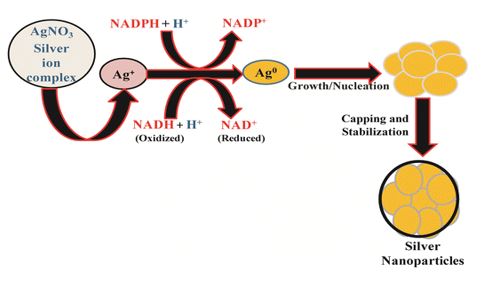
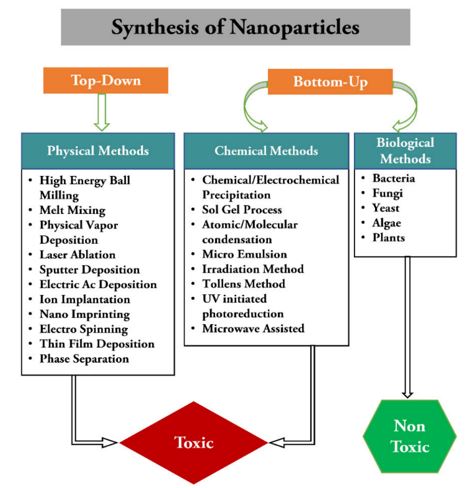
There are several methods for creating nanoparticles, such as chemical, physical, and biological ones. Even while the chemical synthesis approach can produce vast quantities of nanoparticles quickly, it still needs capping agents to stabilize the size of the particles. The chemicals used to synthesize and stabilize nanoparticles are hazardous and produce byproducts that are not environmentally friendly. Consequently, there is a growing need for “green nanotechnology. Vitamins, proteins, and polysaccharides—all biologically active substances found in plant extracts—are crucial in the process that turns silver nitrate into silver nanoparticles.
Plant extracts typically contain a variety of polyphenols, including flavanoids, which are effective reducing agents that can be used to create silver nanoparticles. Because of its versatility and ease of application, plant-based NP green synthesis is currently considered the gold standard among these green biological approaches.
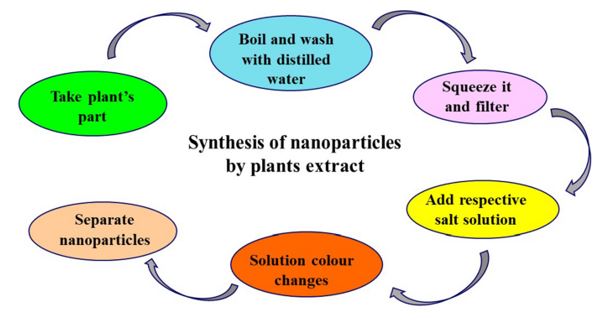
The release of silver ions from the particles, which bestows the antibacterial activity with regard to the microbes, is what gives silver nanoparticles their bacteriocidal characteristics. The nanoparticles have the ability to infiltrate deeply into the cell wall, where they may interact with components that contain phosphorus and sulfur, like DNA and protein, and cause damage to the cell. Secondary metabolites in the plant extract are used for the reduction in the biosynthetic technique, which is environmentally safe and safe.
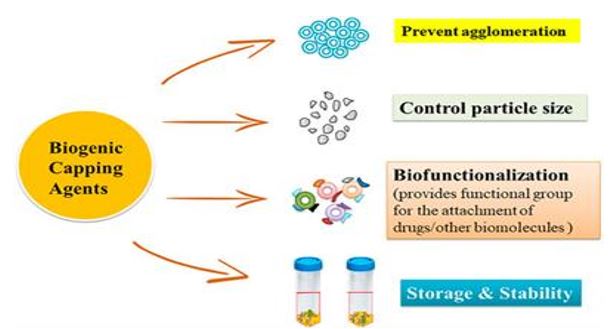
Inspired by the safety-by-design principle, numerous simple, safe, affordable, repeatable, and scalable green synthesis methods for NPs have been developed recently. Because of this, a number of biological systems—including bacteria, yeast, fungus, and plant extracts—are now widely used in green synthesis techniques to produce NPs. The three most crucial conditions for the green synthesis of nanoparticles are the choice of a safe stabilizing material, an appropriate non-toxic reducing agent, and a green or ecologically friendly solvent (water, ethanol, and their combinations are the most often utilized). This environmentally friendly method uses plants, microorganisms, or biological agents as capping and reducing agents. In order to stabilize nanoparticles and stop them from aggregating or coagulating during colloidal synthesis, capping agents are crucial. The interface between the nanoparticles and their preparation medium is stabilized by the capping ligands. Green chemistry-produced silver nanoparticles present a fresh and promising substitute for chemically produced nanoparticles. Green synthesis, then, offers a safe, biocompatible, and ecologically benign way to create NPs for a range of applications, including biomedical ones.
Green Synthesis and Characterization of Silver Nanoparticles
| Plant | Plant part | Technique | Condition | Results | Techniques | Identification | Purpose |
| Aloe barbedensis | Aqueous Extract of leaves | magnetic stirrer | 15 minutes at 65°C | Yellow to reddish brown | UV-Visible spectrophotometer | functional groups | synthesis and stabilization of silver nanoparticles |
| Rubus ellipticus Sm. | Root Extracts | 25 ± 2° C with constant stirring | (FTIR | primary amines group | acts as reducing agent capping and stabilizing agents | ||
| Zeta potential | size distribution | To check the efficiency | |||||
| XRD diffraction | crystallinity | crystalline nature | |||||
| FESEM | surface morphology | spherical morphology | |||||
| EDX analysis | elemental composition | relative abundance of silver, oxygen, carbon, calcium, and chlorine | |||||
| TEM | spherical and monodispersed nanoparticles | shape and size |
Also read: Tour to Microbiology Lab Apparatus
Follow Us on

